Clinical Roadmap
REBALANCE-HF RESPONDER ANALYSIS
Endovascular Ablation of the Right Greater Splanchnic Nerve in Heart Failure with Preserved Ejection Fraction: Initial Responder Analysis
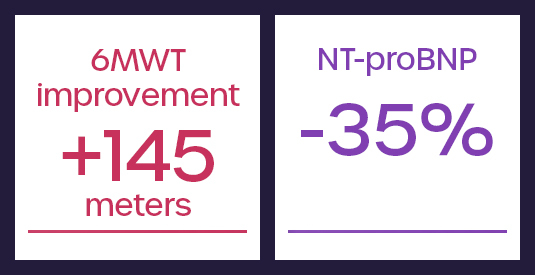
These results from a responder analysis of the REBALANCE-HF randomized feasibility study identified roughly 55% of patients that responded well to the SAVM procedure. These patients saw meaningful improvements in functional capacity, quality of life and reductions in key biomarkers relative to a blinded sham.
View the PresentationREBALANCE-HF PRIMARY RESULTS
Endovascular Ablation of the Right Greater Splanchnic Nerve in Heart Failure with Preserved Ejection Fraction: Primary Analysis of the REBALANCE-HF Randomized Trial
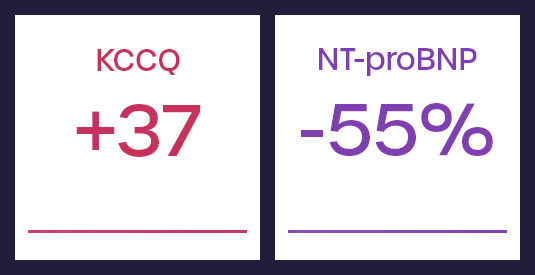
Primary results from the REBALANCE-HF study – the largest, randomized blinded sham-controlled device feasibility study in HFPEF – demonstrate that the procedure is quick and consistent to perform by multiple operators across multiple sites, as well as safe and well tolerated by patients.
View the PresentationREBALANCE-HF 6-MONTH LEAD-IN COHORT
Endovascular Ablation of the Right Greater Splanchnic Nerve in Heart Failure with Preserved Ejection Fraction: Updated Results of the REBALANCE-HF Trial Roll-in Cohort
These results from the roll-in patients of the REBALANCE-HF IDE study support safety and efficacy of the SAVM procedure and signal clinical improvements in key data, including reductions in pulmonary capillary wedge pressure (PCWP) with exercise and improvements in exercise capacity and quality of life.
Read the StudyPilot Endovascular Study in HFrEF
Endovascular Ablation Of The Right Greater Splanchnic Nerve For The Treatment Of HFrEF
These results demonstrate early success using SAVM to treat HFrEF using the Axon Ablation System. Follow-up is ongoing.
See the PosterPresented at HFSA 2021
Pilot Endovascular Study in HFpEF
Durability Of Right Greater Splanchnic Nerve Ablation For The Treatment Of HFpEF
These results demonstrate, for the first time, the feasibility of treating HFpEF with endovascular right-sided GSN ablation. The encouraging safety and efficacy results provide rationale for a phase 2 randomized controlled trial.
See ResultsProcedure Overview
Splanchnic Nerve Modulation in Heart Failure: Mechanistic Overview, Initial Clinical Experience, and Safety Considerations
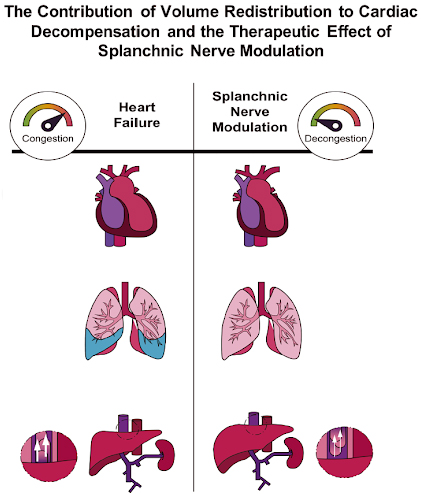
There’s strong basis for continued efforts to study splanchnic nerve therapy in HF provided by the pathophysiological role of the greater splanchnic nerve (GSN) in volume redistribution, the encouraging findings of acute and chronic pilot splanchnic nerve modulation (SNM) studies and the safety profile from permanent SNM for pain.
See the StudyPilot Surgical Study in HFpEF
Surgical Ablation Of The Right Greater Splanchnic Nerve For The Treatment Of HFpEF
In this first-in-human study, GSN ablation in HFpEF proved to be feasible, with a suggestion of reduced cardiac filling pressure during exercise, improved quality of life and exercise capacity.
Read the StudyFURTHER ANALYSIS
Early Hemodynamic Changes following Surgical Ablation of the Right Greater Splanchnic Nerve for the Treatment of Heart Failure with Preserved Ejection Fraction
Demonstrates that GSN ablation results in a significant reduction in pulmonary capillary wedge pressure as soon as 24 hours after the procedure compared to baseline.
Read FindingsEditorial
The Splanchnic Reservoir: An Oasis for Blood Volume in Heart Failure with Preserved Ejection Fraction?
Read the PaperMechanism Overview
Role of Volume Redistribution in the Congestion of Heart Failure
This review highlights the importance of volume shifts in addition to the established concept of sodium and fluid retention as the main drivers of cardiovascular decompensation.
See the Study